More Information
Submitted: February 18, 2025 | Approved: February 27, 2025 | Published: February 28, 2025
How to cite this article: Sami H. Anti-TNFalfa for Treatment of Isolated Gastrocnemius Myositis Related to Crohn’s Disease: A Case Report. Arch Case Rep. 2025; 9(3): 073-076. Available from:
https://dx.doi.org/10.29328/journal.acr.1001130
DOI: 10.29328/journal.acr.1001130
Copyright license: © 2025 Sami H. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Crohn’s disease; Inflammatory myopathies; Gastrocnemius myalgia syndrome; Anti-TNFalfa; Adalimumab; Pathology
Anti-TNFalfa for Treatment of Isolated Gastrocnemius Myositis Related to Crohn’s Disease: A Case Report
Hammi Sami1,2*
1Department of Internal Medicine, Le Mans Hospital, France
2Department of Internal Medicine and Clinical Immunology, Angers University Hospital, France
*Address for Correspondence: Hammi Sami, Department of Internal Medicine, Le Mans Hospital, Department of Internal Medicine and Clinical Immunology, Angers University Hospital, France, Email: [email protected]
Background: Extraintestinal manifestations are prevalent among patients diagnosed with inflammatory bowel disease (IBD). Gastrocnemius Myalgia Syndrome (GMS) is a rare manifestation of IBD. The optimal treatment regimen for GMS remains to be elucidated, and current knowledge of its histological patterns is limited.
Case presentation: We present a case of GMS associated with Crohn’s disease. The patient exhibited an original histological pattern characterized by nerve involvement, and the treatment plan involved the administration of adalimumab, leading to effective management of symptoms while reducing the need for steroids.
Conclusion: The body of knowledge concerning gastrocnemius myalgia syndrome is limited, underscoring the need for a comprehensive international registry to facilitate more accurate diagnosis and management strategies. The use of anti-TNFalfa therapy appears to be a relevant treatment approach.
The aetiology of Inflammatory Bowel Disease (IBD) remains unknown. These disorders are characterized by chronic, intermittent, or persistent inflammation of a portion of the gastrointestinal tract. IBD is comprised of ulcerative colitis, Crohn’s disease, and patterns that are unclassifiable and/or overlapping.
Extraintestinal manifestations (EIM) are observed in approximately 25% of patients with ulcerative colitis and are a common occurrence in Crohn’s disease. The most commonly occurring EIMs involve joints, eyes, skin, liver, and biliary tract [1]. Some manifestations will follow the course of the IBD, for example, peripheral arthritis, whereas others will not, for example, primary sclerosing cholangitis [2].
Additionally, several less prevalent EIMs have been documented, including vascular, neurological, pulmonary, and cardiac involvement [3]. One of the less common EIMs is muscular involvement in the form of Gastrocnemius Myalgia Syndrome (GMS). It presents with calf myalgia and heterogeneous myositis findings on muscle biopsy [4]. Although it is a rare manifestation, with fewer than twenty cases described in the literature, this manifestation appears to offer valuable diagnostic insights, as an association with IBD is consistently reported [4,5]. The management of IBD is contingent upon the severity of the disease and the long-term risk of complications. In cases of mild or severe disease, a therapy based on an anti-TNFα agent (typically infliximab or adalimumab) is typically indicated. A combination with an immunomodulatory agent, such as azathioprine, may be employed [6].
Furthermore, the utilization of anti-TNFalpha agents has been demonstrated to facilitate steroidsparing strategies [7], a pivotal consideration given the deleterious effects associated with prolonged steroid treatment [8]. The selection of an anti-TNF alfa agent is typically guided by patient preferences or prior treatment history, as there is no observed difference in efficacy between adalimumab and infliximab. It is noteworthy that in European countries, infliximab is exclusively administered intravenously [9].
This report presents a case of GMS associated with Crohn’s disease, which displays an original histological finding and the follow-up after a six-month course of subcutaneous adalimumab-based monotherapy. Subsequently, we examine the clinical and histological characteristics of GMS.
History of present illness
The patient was initially admitted to the hospital for abdominal pain associated with grossly bloody diarrhea and an absence of fever.
A physical examination revealed a soft and non-tender abdomen, with the hypogastric region painful to palpation. The remainder of the examination was unremarkable.
Laboratory examinations showed a C-reactive protein (CRP) level of 291 mg/L and a neutrophil leukocytosis of 14.8 G/L. Faecal calprotectin levels were measured at 2542 mg/kg. Stool culture results revealed no enteropathogenic bacteria and testing for Clostridium difficile was negative.
Rectosigmoidoscopic investigation showed mild inflammation and swelling of the sigmoid and rectal mucosa.
A Computed Tomography (CT) scan of the abdomen showed ileitis and colitis.
A ceftriaxone-metronidazole regimen was administered, resulting in partial symptom relief, with persistent abdominal pain and diarrhea persisting a month later.
Second hospitalization
The patient was readmitted to the hospital one week later, in the same month, with bloody diarrhea, accompanied by inflammatory pain in the bilateral calves.
Laboratory examinations revealed a C-reactive protein (CRP) level of 226 mg/L and neutrophil leukocytosis (12 G/L). Creatine kinase levels were within the normal range. Antineutrophil Cytoplasmic Antibodies (ANCA) were tested negative by indirect immunofluorescence.
The same microbial investigations, as during the initial hospitalization, were again negative or sterile.
An upper endoscopy was performed, revealing no histologic abnormalities. An ileocolonoscopy revealed three millimetric ulcers in the terminal ileum.
Histological analysis indicated epithelial inflammation with ulcers and cecum inflammation.
A CT scan of the abdomen revealed ileocecitis associated with discrete pelvic effusion and numerous ileal lymph nodes.
The patient was treated with opioid analgesics and was discharged after the spontaneous resolution of the diarrhea. She underwent a 48-hour course of tinzaparin until deep vein thrombosis of the lower limb was ruled out by Doppler ultrasonography.
Third hospitalization
The patient was readmitted to the hospital one month later, with symptoms including abdominal pain, grossly bloody diarrhea, and bilateral calf pain.
The patient exhibited elevated C-reactive protein (CRP) levels of 226 mg/L and a neutrophil count of 8.6 G/L.
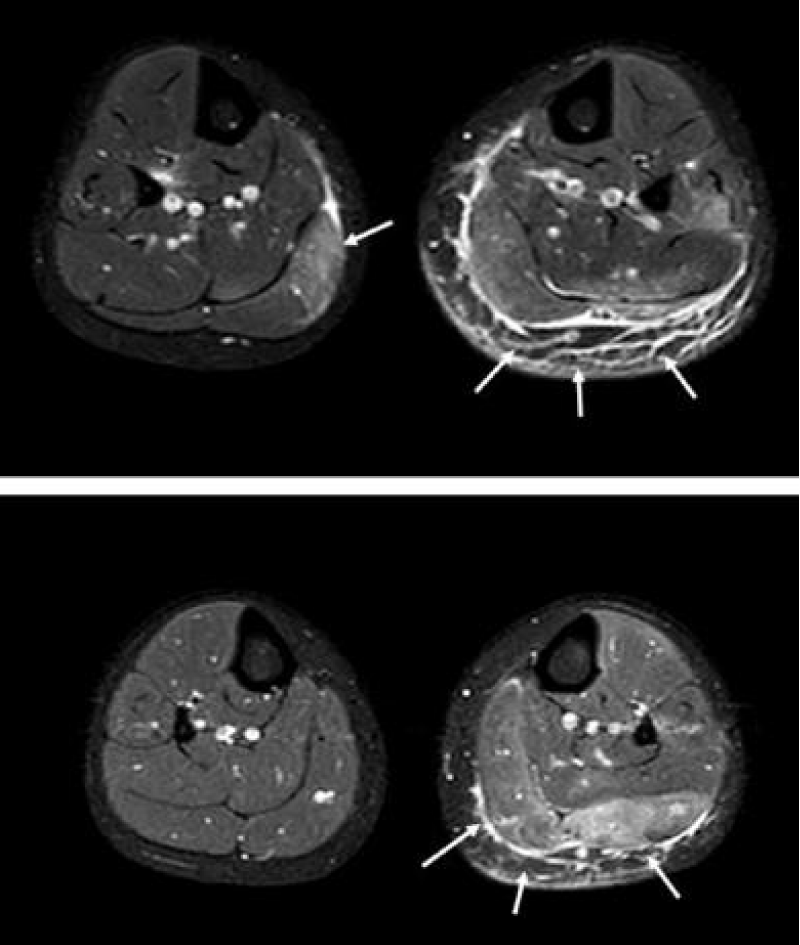
Consequently, Magnetic Resonance Imaging (MRI) of the lower limbs was performed, revealing a T2 Short T1 Inversion Recovery (STIR) hypersignal in the bilateral gastrocnemius medial muscles and soleus muscles, as well as in the left gastrocnemius muscle (Figure 1).
Concurrently, antinuclear antibody testing, employing the indirect immunofluorescence method on HEp-2 cells, yielded a negative result. No auto-immune hepatitis-associated antibodies or type 2 antimitochondrial antibodies were identified. An isolated anti-cN1A antibody was identified, with no additional myositis-associated antibodies detected.
Muscle biopsies revealed a discrete infiltrate made of lymphoplasmocytic cells in the perimysium and around a capillary. No granuloma, vasculitis, or necrosis was identified.
The patient was administered a methylprednisolone dosage of 0.8 mg/kg intravenously over a five-day period, in conjunction with an antibiotherapy course comprising ceftriaxone and metronidazole.
The therapeutic interventions led to a swift alleviation of symptoms, including leg pain and diarrhea.
Past medical history
The patient had a medical history of an ovarian cyst that was surgically removed.
Conclusion of hospitalizations
A GMS associated with Crohn’s disease diagnosis was retained by a multidisciplinary team (clinical immunologists, gastroenterologists, rheumatologists, and infectious disease specialists).
Adalimumab treatment was initiated subcutaneously in accordance with the following protocol: Initially, 160 mg was administered, followed by 80 mg in the subsequent week and 40 mg every two weeks.
Outcome and follow-up
The patient’s response to treatment was evaluated at the six-month follow-up visit, at which time the patient exhibited no signs of active disease, as indicated by the absence of calf pain or diarrhea relapse. No steroid therapy course was deemed necessary. The patient’s weight returned to normal. Laboratory examinations revealed a C-reactive protein (CRP) level of 1.6 mg/L and a neutrophil leukocytosis of 8.2 G/L.
Figure 1: Magnetic resonance Imaging of the calves in axial view on STIR-weighted images. The arrows indicate the hypersignal reflecting inflammatory myopathy.
The prevalence of muscular involvement, particularly inflammatory myopathies, in patients with Inflammatory Bowel Diseases (IBDs) remains unclear. A more comprehensive understanding of this condition is essential for enhancing diagnosis and therapeutic management. We present a case of GMS associated with Crohn’s disease, highlighting an unreported nerve involvement, and the second case demonstrating the efficacy of adalimumab in achieving quiescence and reducing steroid use.
The clinical features of our case are consistent with earlier observations [5]. The features that appear the most frequently in GMS are female sex, simultaneous occurrence of intestinal and muscular manifestations, normal CK serum level, and steroid dependency or resistance in almost half of the cases (Table 1).
| Table 1: Clinical features of seventeen GMS associated with Crohn's disease. Adapted from Reference 5. | ||||||
| n (%) | ||||||
| Age | Intestinal | Elevated | Steroid- Need for anti-TNF | |||
| (Median [Quartile]) |
Sex (female) | Manifestations at the time of GMS | CK serum level | Dependence or resistance | Alpha for remission achievement | IFX/ADA |
| 26 [21-33] | 10 (59) | 15 (88) | 2 (12) | 8 (47) | 7 (44) | 5/2 |
The most common histopathological pattern observed is non-granulomatous non-vasculitis myositis (Table 2), which is consistent with the findings in our case. To the best of our knowledge, there has been no prior report of nerve involvement in conjunction with GMS. It is noteworthy that the patient exhibited no symptoms of peripheral neuropathy and had no history of peripheral nerve disease, such as lower limb radiculopathy. However, the presence of peripheral nerve involvement can be indicative of either a normal variant or a lack of specificity [10]. Consequently, further investigation is necessary to ascertain the relationship, if any, between this histological finding and GMS. An electroneuromyography is planned for our patient to shed more light on the matter.
| Table 2: Histopathological findings on thirteen muscle biopsies. Adapted from Reference 5. | ||
| n (%) | ||
| Granulomatous myositis | 3 (23) | |
| Vasculitis | 5 (38) | |
| Necrotizing | 2 (15) | |
| Non-necrotizing | 3 (23) | |
| Non-granulomatous non-vasculitis myositis | 10 (77) | |
Conventional management of inflammatory myopathies does not include the use of anti-TNFalpha agents [11]. However, as a recommended biological agent therapy in IBDs, almost half of the patients in our case study have received either infliximab or adalimumab (Table 1). In our case, adalimumab administration resulted in sustained remission of muscle and gastrointestinal manifestations at the six-month follow-up, without the need for steroid reliance. The rationale underlying this choice is rooted in the known parallel courses of EIM and gastrointestinal manifestations in IBDs, suggesting a shared physiopathological mechanism.
As previously posited by other researchers [12], the occurrence of such muscular involvement may be attributable to the homing of antigen-stimulated lymphocytes. This pathogenic pathway has the potential to elucidate the preferential perimysium and capillary infiltrates observed.
We hereby present a novel case of Gastrocnemius Myalgia Syndrome (GMS) associated with Crohn’s disease. As a rare Extraintestinal Manifestation (EIM), we have augmented the existing body of knowledge concerning the clinical and histopathological features of GMS. Moreover, our case demonstrates the efficacy of adalimumab in achieving remission, thereby supporting the hypothesis of a parallel course and a shared physiopathological mechanism between GMS and associated Inflammatory Bowel Disease (IBD).
Consent: A formal oral consent was obtained from the patient for publication.
LEGENDRE Paul and LOZAC’H Pierre, medical doctors, for supervising the manuscript.
GUERZIDER Antoine, medical doctor for assisting in data collection.
- Gros B, Kaplan GG. Ulcerative Colitis in Adults: A Review. JAMA. 2023;330(10):951-65. Available from: https://doi.org/10.1001/jama.2023.15389
- Generini S, Giacomelli R, Fedi R, Fulminis A, Pignone A, Frieri G, et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: an open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63(12):1664 9. Available from: https://doi.org/10.1136/ard.2003.012450
- Das KM. Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig Dis Sci. 1999;44(1):1-13. Available from: https://doi.org/10.1023/a:1026629528233
- Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1915-1924. Available from: https://doi.org/10.1002/ibd.20942
- Catherine J, Kadhim H, Lambot F, Liefferinckx C, Meurant V, Otero Sanchez L. Crohn's disease-related 'gastrocnemius myalgia syndrome' successfully treated with infliximab: A case report. World J Gastroenterol. 2022;28(7):755-762. Available from: https://doi.org/10.3748/wjg.v28.i7.755
- Dassopoulos T, Sultan S, Falck-Ytter YT, Inadomi JM, Hanauer SB. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145(6):1464-78.e1-5. Available from: https://doi.org/10.1053/j.gastro.2013.10.046
- Akobeng AK, Zachos M. Tumor necrosis factor-alpha antibody for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2004;2003(1):CD003574. Available from: https://doi.org/10.1002/14651858.cd003574.pub2
- Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;2008(2):CD006792. Available from: https://doi.org/10.1002/14651858.cd006792.pub2
- Osterman MT, Haynes K, Delzell E, Zhang J, Bewtra M, Brensinger C, Chen L, Xie F, Curtis JR, Lewis JD. Comparative effectiveness of infliximab and adalimumab for Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(5):811-817.e3. Available from: https://doi.org/10.1016/j.cgh.2013.06.010
- Chkheidze R, Pytel P. What Every Neuropathologist Needs to Know: Peripheral Nerve Biopsy. J Neuropathol Exp Neurol. 2020 Apr 1;79(4):355-364. Available from: https://doi.org/10.1093/jnen/nlaa012
- Troyanov Y, Targoff IN, Tremblay JL, Goulet JR, Raymond Y, Senécal JL. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies: analysis of 100 French Canadian patients. Medicine (Baltimore). 2005;84(4):231-249. Available from: https://doi.org/10.1097/01.md.0000173991.74008.b0
- Christopoulos C, Savva S, Pylarinou S, Diakakis A, Papavassiliou E, Economopoulos P. Localised gastrocnemius myositis in Crohn's disease. Clin Rheumatol. 2003;22(2):143-5. Available from: https://doi.org/10.1007/s10067-002-0679-9