More Information
Submitted: March 25, 2025 | Approved: April 08, 2025 | Published: April 09, 2025
How to cite this article: Yenikepalli L, Rukmini Reddy G, Srinivas Reddy M, Srinivasulu Reddy B. Anesthetic Management of Patient with Asymptomatic Stuck Mitral Valve (Prosthetic Heart Valve Thrombosis) Case Posted for LSCS – Case Report. Arch Case Rep. 2025; 9(4): 135-139. Available from:
https://dx.doi.org/10.29328/journal.acr.1001134
DOI: 10.29328/journal.acr.1001134
Copyright license: © 2025 Yenikepalli L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Anesthetic Management of Patient with Asymptomatic Stuck Mitral Valve (Prosthetic Heart Valve Thrombosis) Case Posted for LSCS – Case Report
Lavanya Yenikepalli1, Rukmini Reddy G2, Srinivas Reddy M3 and Srinivasulu Reddy B3*
1DNB Anesthesia, Fellowship in Onco-Anesthesia, Junior Consultatant, Malla Reddy Narayana Multispecilaty Hospital, India
2MD Anesthesia, Senior consultant at Malla Reddy Narayana Multispecilaty Hospital, India
3DNB Anesthesia, PDCC in Cardio-thoracic Anesthesia, Senior Consultatant at Malla Reddy Narayana Multispecilaty Hospital, India
*Address for Correspondence: Dr. Srinivasulu Reddy B, Department of Anesthesia, 3rd floor, OT complex, Malla Reddy Narayana Multispecilaty Hospital, Jeedimetla, Hyderabad, Telangana 500055, India
Rheumatic fever is the most common cause of mitral stenosis. Two-thirds of all patients with rheumatic mitral stenosis are females. Pregnancy is poorly tolerated in patients with MS, and female patients with MS become symptomatic during pregnancy because of hemodynamic changes like increase in intravascular volume, blood pressure and heart rate, which worsens NYHA grade to another [1]. Patients who are not suitable for percutaneous balloon mitral valvotomy should undergo mitral valve replacement (MVR) surgery either with mechanical or bioprosthetic valve [2]. Bioprosthetic valve is advisable in women of child bearing age group [3,4]. As pregnancy is a hypercoagulable state, anticoagulation becomes more challenging. Risk of maternal and fetal complications can occur due to mechanical prosthetic valves. Hypercoagulable state of pregnancy and inadequate anticoagulation may lead to prosthetic valve thrombosis (PVT) or stuck mitral valve. Now we are presenting anaesthetic challenges in a pregnant patient for lscs with prosthetic valve thrombosois as such similar case was not reported previously.
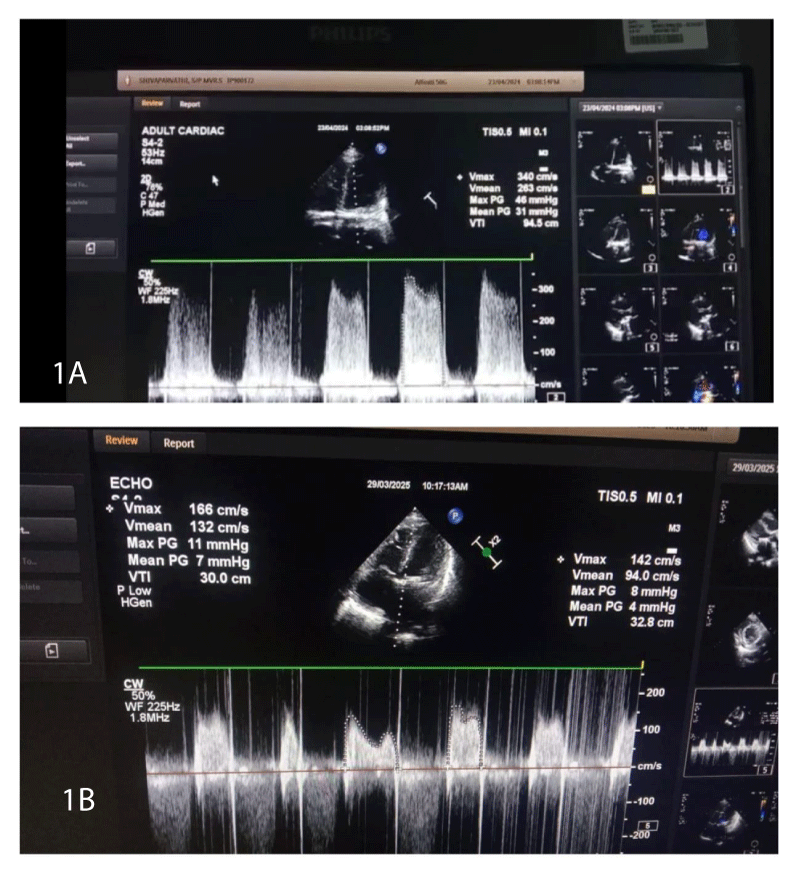
A 29 year old female G2P1L1, who is a known case of hypothyroidism and chronic rheumatic heart disease, underwent MVR 10 years ago. Patient was on warfarin therapy and bridged with low molecular weight heparin (LMWH) 40 mg once daily dosing (elsewhere) after conception. (Which is inadequate to prevent thrombosis in a patient with MVR). Missed her antenatal visits for 4 months, presented to our hospital Obstetrician in the seventh month of pregnancy. All relevant investigations were done, 2D Echo revealed subvalvular thrombus size 1.1 X 1.0 cms, mitral valve gradient - 38/24 mm of Hg (Figure 1), dilated LA size of 4.8 cm, mild PAH with good LV function. Patient was asymptomatic and was on anticoagulants, antiplatelets, diuretics, and beta-blockers as advised by cardiologist. Baseline Prothrombin Time/ International Normalized Ratio (PT/INR) -13.8/0.98, Aptt-32.6, platelet count - 2,30,000 per microliter.
Figure 1: Gradient across mitral valve area. 1A: high gradient across the mitral valve area preoperatively 1B: decreased gradient across the mitral valve area postoperatively due to thrombus resolution
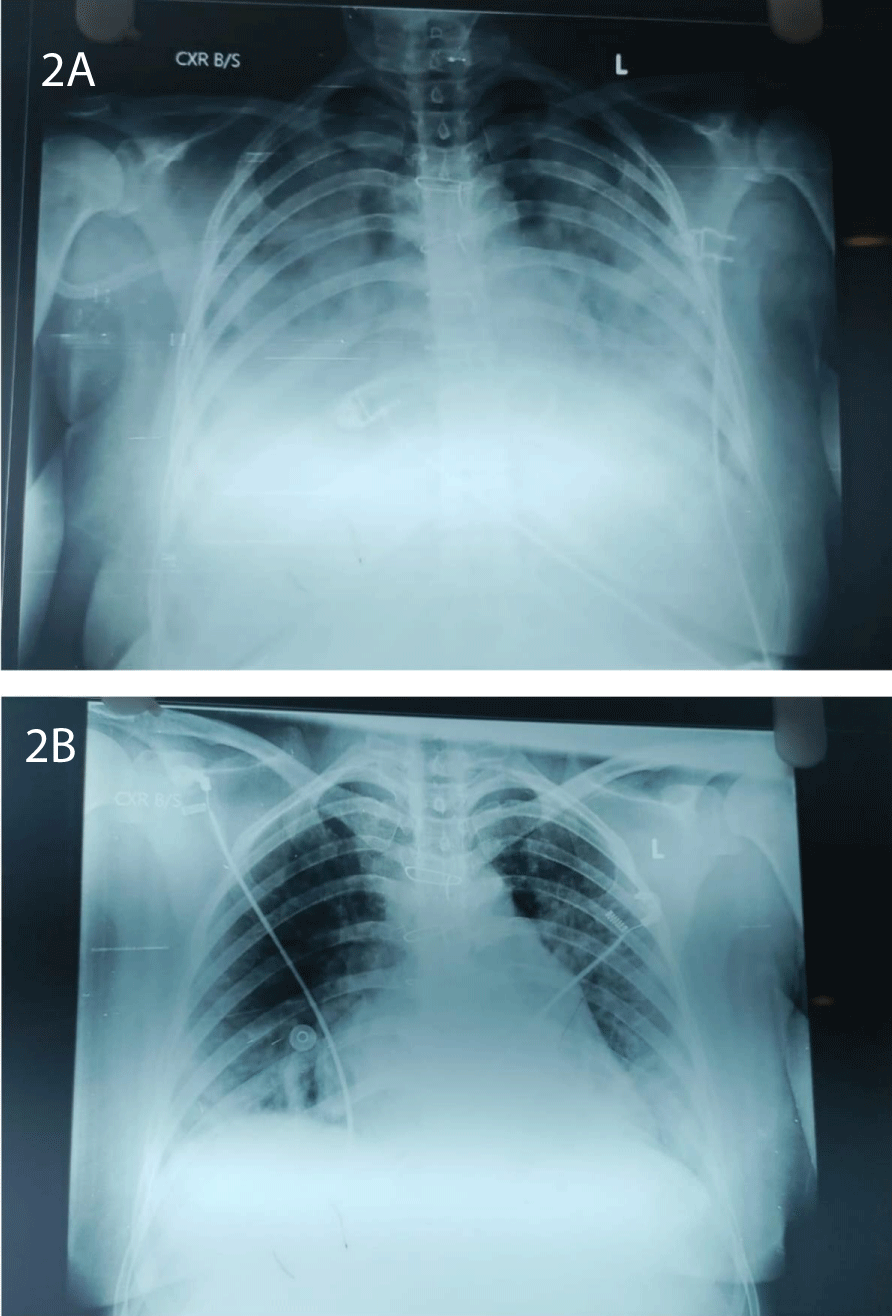
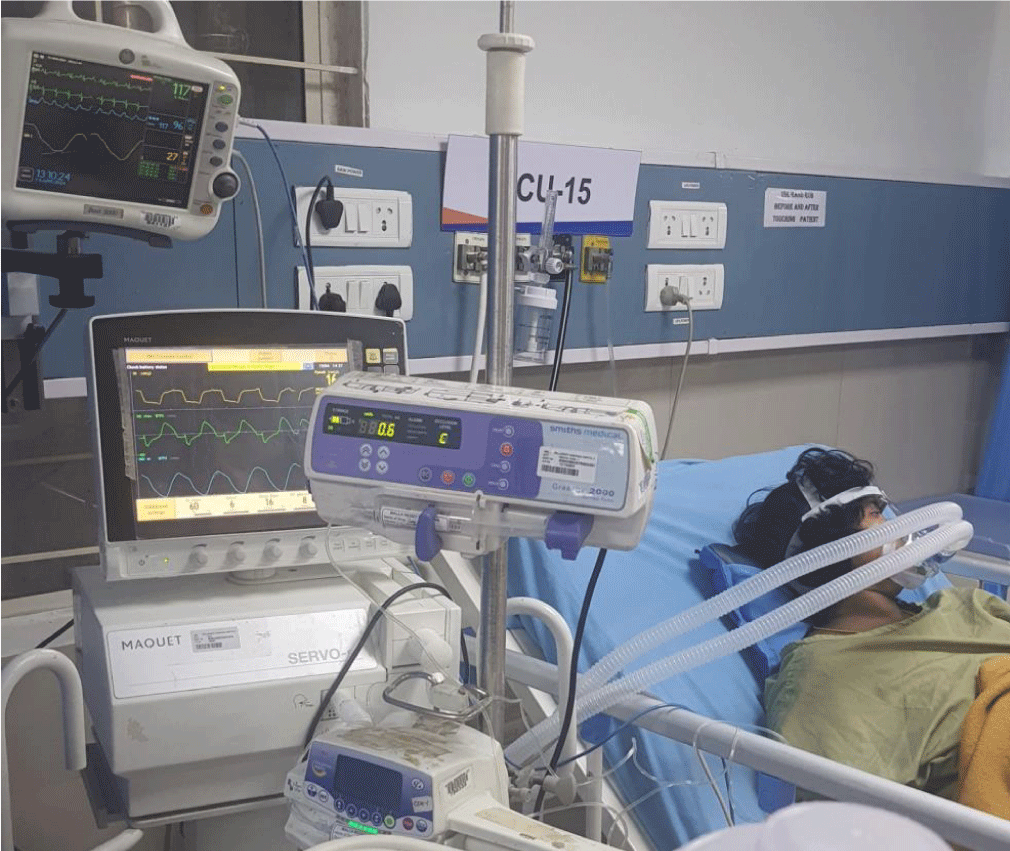
Patient came to Pre Anesthetic Checkup (PAC) for the first time at 37 weeks of gestation (WOG), 2D Echo revealed a prosthetic valve thrombus measuring 0.8 × 0.7 cm. Patient and her family members explained about necessity for redo-MVR and risk of sudden decompensation as they did not give consent for MVR and want to go ahead with elective LSCS. After multidisciplinary team approach and Informed and written consent, Patient was posted for elective LSCS. Intraoperatively ASA standard monitoring was done. Prophylactic antibiotic was given to prevent infective endocarditis. Before inducing the patient, arterial and central venous lines secured, left radial artery cannulated with 20G switch cannula, right internal jugular vein, with 7 French, 13 cms., and cardiothoracic team was informed and cardiopulmonary bypass setup was prepared in advance. Patient was induced with etomidate 0.3 mg/kg, Rapid sequence induction was performed using rocuronium 1.2 mg/kg as pregnancy is full stomach condition. Intubation response blunted with IV lignocaine and trachea was intubated with 7 mm ETT, fixed at 19 cms after five-point auscultation. Anesthesia was maintained with TIVA propofol before the baby’s delivery, and fentanyl at a dose of 2 mcg/kg was given immediately after baby and cord clamping. Throughout the case anesthesia maintained with 0.6 MAC of sevoflurane. A slow bolus of 3 units of oxytocin was administered, followed by a continuous infusion. Precipitous fall in blood pressure was associated with bolus dose of oxytocin, nor-adrenaline infusion was started and titrated according to BP to maintain a MAP of 60 - 70 mmHg. Vitals were well maintained throughout the surgery. The patient was extubated on the operating table and shifted to surgical intensive care unit in hemodynamically stable condition. Postoperatively 2D echo, ECG, and cardiologist review was done. Patient was started on anti-coagulants and anti-failure medication. On second postoperative day patient developed pulmonary edema. Patient suddenly desaturated to 82% on room air. Arterial blood gas revealed hypoxemia, and chest X-ray showed a typical bat-wing appearance (Figure 2). Patient kept in propped up position, noninvasive-positive pressure ventilation was initiated CPAP mode with PEEP OF 6 cmH2O, PC above PEEP of 8 cmH2O (Figure 3). Injection furosemide of 0.6 ml/hour infusion was initiated equivalent to 1 mg per 0.1 mL. Pulmonary edema resolved after 36 hours of treatment and patient was discharged home on fifth postoperative day. Complete resolution of the thrombus noted in the subsequent follow-ups.
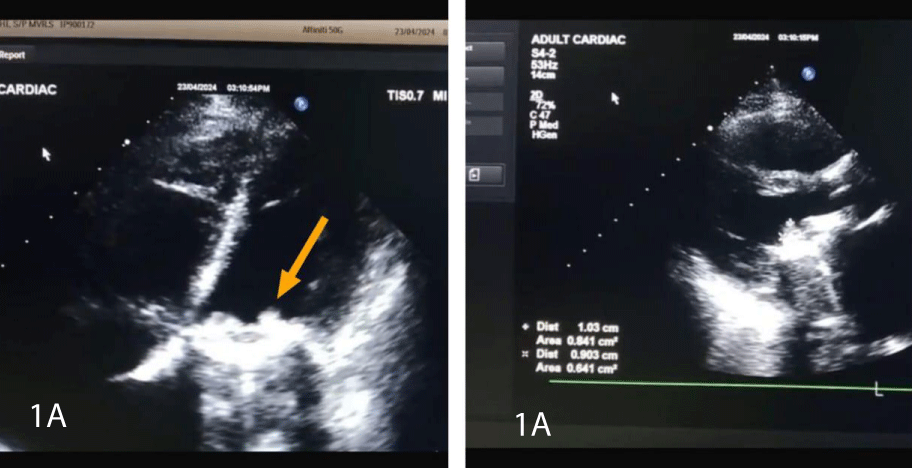
Figure 1A: BrainFigure 1A: Image showing Subvaluvlar Thrombus.
Figure 2: A: typical Bat wing appearance; Figure 2B: resolving phase of Pulmonary Edema
Figure 3: Patient on NIPPV support..
According to Virchow’s triad [3,5], endovascular thrombus formation involves 3 main mechanisms i.e.
Surface - healthy endothelium resists thrombosis, whereas thrombogenic property is seen with intravascular prosthetic material.According to Virchow’s triad [3,5], endovascular thrombus formation involves 3 main mechanisms i.e.
Surface - healthy endothelium resists thrombosis, whereas thrombogenic property is seen with intravascular prosthetic material.
Hemodynamic factors – mechanical valves create an abnormal flow conditions, zones of high shear stress which can cause platelet activity.
Hemostatic factors - hypercoagulable state, Which necessitates the patients with mechanical prosthetic valve for lifelong anticoagulation with Vitamin K antagonists (VKA) (hemodynamic and physical properties of mechanical valve remains thrombogenic). And, it is recommended to maintain an International Normalized Ratio (INR) within the range of 2.5 to 3.5 in order to minimize the risk of complications [3].
Pregnant patients receiving warfarin between sixth to ninth WOG is associated with warfarin fetopathy and warfarin embryopathy, after 36 weeks of gestation warfarin is associated with intracranial hemorrhage (with normal vaginal delivery). Warfarin embryopathy risk is low if warfarin dose is < _5 mg/day [6,7]. American Heart Association/American College of Cardiology guidelines (AHA/ACC guidelines) recommend use of oral anticoagulants in second and third trimester until approximately 36 WOG, because warfarin carries lowest mechanical valve thrombosis and death. Provided INR is strictly maintained. Patient is bridged with LMWH (adequate anticoagulation maintained with anti Xa of 0.8-1.2U) or UFH (maintained aPTT*2).
Pregnancy is hypercoagulable state and marked hemodynamic changes during pregnancy and /or inadequate anticoagulation make pregnant more prone to PVT, obstructive left sided mechanical heart valve thrombus (presents with acute decompensation and acute pulmonary edema), recurrent valve thrombosis (thrombus size of >10 mm [4] requires emergency fibrinolysis/emergency surgery [3,8]). Non-obstuctive type thrombus size of <0.8 cm2 and NYHA I-II, in this situation heparin is recommended for initial management [5,8]. (In patients with mechanical MVR presents with stroke or systemic emboli, while in therapeutic range of VKA, increase INR target to 4.0 or add a daily low dose aspirin 75 to 100 mg3).
J.M. Gonzulcz-Santos. J.L. Vallejo. M.L Rico, et al. [9] and Tempe, et al. [10] Keeping these case reports in mind we proceeded with LSCS only because patient and her relatives did not give consent for elective MVR. As MV orifice decreases, left atrium dilates and left atrial pressure increase, because of which a pressure gradient develops between left atrium and left ventricle. This pressure gradient during diastole is the hemodynamic hallmark of MS. PVT/ stuck valve patients behaves like fixed cardiac output states such as mitral stenosis (MS) (Table 1).
| Table 1 | |||||
| Hemodynamic parameter |
Hemodynamic goal | Physiological changes during pregnancy | Drawback | Precaution/ treatment | |
| 1 | Heart rate and Rhythm | Maintain 80- 100bpm. Normal sinus rhythm | HR increases during 1st trimester of pregnancy. Pain ,anxiety during labor- catecholamine release results in tachycardia | Diastolic filling time deceases, CO deceases, backflow increases &leads to pulmonary edema |
Beta blocker- risk of IUGR present |
| 2 | Preload | Judicious fluid loading Avoid fluid overload | Plasma and blood volume increases | Risk of fetoplacental circulatory insufficiency and acute decomposition | Diuretic therapy3 |
| Aorto-caval compression in supine position – decreases preload12 | 300 left tilt , left lateral decubitus position |
||||
| 3 | Afterload | Maintain afterload | Afterload decreases in early pregnancy – Hormones(E&P), low resistance uteroplacental circulation Oxytocin drip 13 |
Decreases coronary perfusion and aortic diastolic pressure and increases LV enddiastolic pressure11 | Control sudden fall in afterload Oxytocin-slow 3U bolus followed by 18-36U/hr13 Use of vasopressors |
| 4 | Pulmonary vascular resistance | Maintain PVR | PVR decreases because of hormones | Avoid hypoxia, hypercarbia, acidosis, methergine, carboprost -causes PVH | |
| Volume overload right after delivery13 | Diuretic usage | ||||
Tranexamic acid better be avoided in patients with a known contraindication to ant fibrinolytic therapy such as thromboembolic disease during pregnancy. Transvalvular gradient increases in pregnant women as compared to non pregnant woman because of increased CO and HR.
Rapid sequence induction is done as pregnancy is full stomach condition, care was taken to attenuate hemodynamic response during laryngoscopy and intubation. (Severe cardiac disease patients along with standard ASA monitoring invasive arterial blood pressure monitoring, central venous access is recommended if expected to use vasopressor or inotropic agents. in high-risk non-cardiac surgeries, a cardiopulmonary bypass setup should be on standby).
During the perioperative period, the mother is at increased risk of developing pulmonary edema and experiencing arrhythmias. Risk of pulmonary edema is highest immediately postpartum due to auto-transfusion, which significantly increases preload of 400-900ml of blood which fills central circulation, which continues to occur for 24- 72 hours post-delivery [1] usually treated with positioning, oxygen therapy, diuretics, NIPPV and fluid restriction [14].
Hemodynamic factors – mechanical valves create an abnormal flow conditions, zones of high shear stress which can cause platelet activity.
Hemostatic factors - hypercoagulable state, Which necessitates the patients with mechanical prosthetic valve for lifelong anticoagulation with Vitamin K antagonists (VKA) (hemodynamic and physical properties of mechanical valve remains thrombogenic). And, it is recommended to maintain an International Normalized Ratio (INR) within the range of 2.5 to 3.5 in order to minimize the risk of complications [3].
Pregnant patients receiving warfarin between sixth to ninth WOG is associated with warfarin fetopathy and warfarin embryopathy, after 36 weeks of gestation warfarin is associated with intracranial hemorrhage (with normal vaginal delivery). Warfarin embryopathy risk is low if warfarin dose is <_5 mg/day [6,7]. American Heart Association/American College of Cardiology guidelines (AHA/ACC guidelines) recommend use of oral anticoagulants in second and third trimester until approximately 36 WOG, because warfarin carries lowest mechanical valve thrombosis and death. Provided INR is strictly maintained. Patient is bridged with LMWH (adequate anticoagulation maintained with anti Xa of 0.8-1.2U) or UFH (maintained aPTT*2).
Pregnancy is hypercoagulable state and marked hemodynamic changes during pregnancy and /or inadequate anticoagulation make pregnant more prone to PVT, obstructive left sided mechanical heart valve thrombus (presents with acute decompensation and acute pulmonary edema), recurrent valve thrombosis (thrombus size of >10 mm [4] requires emergency fibrinolysis/emergency surgery [3,8]). Non-obstuctive type thrombus size of <0.8 cm2 and NYHA I-II, in this situation heparin is recommended for initial management [5,8]. (In patients with mechanical MVR presents with stroke or systemic emboli, while in therapeutic range of VKA, increase INR target to 4.0 or add a daily low dose aspirin 75 to 100 mg3).
J.M. Gonzulcz-Santos. J.L. Vallejo. M.L Rico, et al. [9] and Tempe, et al. [10] Keeping these case reports in mind we proceeded with LSCS only because patient and her relatives did not give consent for elective MVR. As MV orifice decreases, left atrium dilates and left atrial pressure increase, because of which a pressure gradient develops between left atrium and left ventricle. This pressure gradient during diastole is the hemodynamic hallmark of MS. PVT/ stuck valve patients behaves like fixed cardiac output states such as mitral stenosis (MS) (Table 1).
Tranexamic acid better be avoided in patients with a known contraindication to ant fibrinolytic therapy such as thromboembolic disease during pregnancy. Transvalvular gradient increases in pregnant women as compared to non pregnant woman because of increased CO and HR.
Rapid sequence induction is done as pregnancy is full stomach condition, care was taken to attenuate hemodynamic response during laryngoscopy and intubation. (Severe cardiac disease patients along with standard ASA monitoring invasive arterial blood pressure monitoring, central venous access is recommended if expected to use vasopressor or inotropic agents. in high-risk non-cardiac surgeries, a cardiopulmonary bypass setup should be on standby).
During the perioperative period, the mother is at increased risk of developing pulmonary edema and experiencing arrhythmias. Risk of pulmonary edema is highest immediately postpartum due to auto-transfusion, which significantly increases preload of 400-900ml of blood which fills central circulation, which continues to occur for 24- 72 hours post-delivery [1] usually treated with positioning, oxygen therapy, diuretics, NIPPV and fluid restriction [14].
Pregnancy with a prosthetic heart valve represents a high-risk clinical scenario with increased maternal and fetal morbidity. Continuous monitoring and thorough risk stratification are needed to prevent complications However use of low dose vitamin k antagonist and targeted substitution with parental heparin has reduced maternal and fetal adverse outcomes. These patients are best managed in tertiary care centres with a team comprising the obstetrician, cardiologist, hematologist, neonatologist and intensivists. Proper preoperative preparation and multidisciplinary approach is crucial for patient outcome.
Ethical approval and consent to publish
The authors confirm that written informed consent was obtained from the patient for the publication of this case report and any accompanying images. Ethical approval was not required for this single-patient case report, as per institutional guidelines.
- Kannan M, Vijayanand G. Mitral stenosis and pregnancy: current concepts in anaesthetic practice. Indian J Anaesth. 2010;54(5):439–44. Available from: https://doi.org/10.4103/0019-5049.71043
- Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation. 2006;114(5):e84–231. Available from: https://doi.org/10.1161/circulationaha.106.176857
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary. Circulation. 2021;143(5):e35–71. Available from: https://doi.org/10.1161/cir.0000000000000932
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. Available from: https://doi.org/10.1093/eurheartj/ehab395
- Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic heart valve thrombosis. J Am Coll Cardiol. 2016;68(24):2670–89. Available from: https://doi.org/10.1016/j.jacc.2016.09.958
- Libby P, Zipes DP, Bonow RO, Mann DL, Tomaselli GF. Pregnancy and heart disease. In: Braunwald E, editor. Braunwald's heart disease: a textbook of cardiovascular medicine. 11th ed. Philadelphia: Elsevier; 2018;1788.
- van Hagen IM, Roos-Hesselink JW, Ruys TP, Merz WM, Goland S, Gabriel H, et al. Pregnancy in women with a mechanical heart valve: data of the ESC Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132(2):132–42. Available from: https://doi.org/10.1161/circulationaha.115.015242
- Özkan M, Çakal B, Karakoyun S, Gürsoy OM, Çevik C, Kalçık M, et al. Thrombolytic therapy for prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tPA. Circulation. 2013;128(5):532–40. Available from: Available from: https://doi.org/10.1161/circulationaha.113.001145
- González-Santos JM, Vallejo JL, Rico MJ, González-Santos ML, Horno R, García-Dorado D. Thrombosis of a mechanical valve prosthesis late in pregnancy: case report and literature review. Thorac Cardiovasc Surg. 1986;34(5):335–7. Available from: https://doi.org/10.1055/s-2007-1022166
- Tempe DK, Virmani S, Tempe A, Sharma JB, Nigam M. Anaesthetic management of emergency caesarean section and reoperative mitral valve replacement at 32 weeks: case report. Ann Card Anaesth. 2002;5(1):63–7. Available from: https://pubmed.ncbi.nlm.nih.gov/17890804/
- Meng ML, Arendt KW, Banayan JM, Bradley EA, Vaught AJ, Hameed AB, Anesthetic care of the pregnant patient with cardiovascular disease: AHA scientific statement. Circulation. 2023;147(11):e657–73. Available from: https://doi.org/10.1161/cir.0000000000001121
- Kuo CD, Chen GY, Yang MJ, Tsai YS. Effect of position on autonomic nervous activity in late pregnancy. Anaesthesia. 1997;52(12):1161–5. Available from: https://doi.org/10.1111/j.1365-2044.1997.254-az0387.x
- Girnius A, Meng ML. Cardio-obstetrics: a review for the cardiac anesthesiologist. J Cardiothorac Vasc Anesth. 2021;35(12):3483–8. Available from: https://doi.org/10.1053/j.jvca.2021.06.012
- Purvey M, Allen G. Managing acute pulmonary oedema. Aust Prescr. 2017;40(2):59–63. Available from: https://doi.org/10.18773/austprescr.2017.012