More Information
Submitted: 02 November 2019 | Approved: 05 December 2019 | Published: 06 December 2019
How to cite this article: Salvatore D, Daniela M, Antonio D, Marisa M, Roberto C, et al. Scraping cytology and scanning electron microscopy in diagnosis and therapy of corneal ulcer by mycobacterium infection. Arch Case Rep. 2019; 3: 050-053.
DOI: 10.29328/journal.acr.1001024
Copyright License: © 2019 Salvatore D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Scraping cytology and scanning electron microscopy in diagnosis and therapy of corneal ulcer by mycobacterium infection
Del Prete Salvatore1, Marasco Daniela1, Del Prete Antonio2, Meloni Marisa3, Capaldi Roberto2, Grumetto Lucia4 and Russo Giacomo4*
1Service Biotech, srl. 80132 Naples, Italy
2Department of Neurosciences and Reproductive and Dentistry Sciences, University of Naples Federico II, 80131 Naples, Italy
3Vitroscreen srl. 20149 Milan, Italy
4Pharm-Analysis & Bio-Pharm Laboratory, Department of Pharmacy, School of Medicine and Surgery, University of Naples Federico II, Via D. Montesano, 49, I-80131 Naples, Italy
*Address for Correspondence: Giacomo Russo, Pharm-Analysis & Bio-Pharm Laboratory, Department of Pharmacy, School of Medicine and Surgery, University of Naples Federico II, Via D. Montesano, 49, I-80131 Naples, Italy, Tel: +39 081678639; Email: giacomo.russo@unina.it
Purpose: This work is aimed at demonstrating that scraping cytology and scanning electron microscopy can successfully assist in the diagnosis of nontuberculous mycobacteria infection. For this purpose, we report the use of both these techniques in the diagnosis of cornel ulcer in a previously healthy young man.
Methods: Cytological samples were achieved by scraping technique on the mucosa, both sub palpebral and temporal area of the eye tarsal conjunctiva. The obtained sample was affixed to a sanded rectangular slide, stained with the Pappenheim method, washed in bidistilled water, treated in Giemsa solution, washed again and subsequently dried on a hot plate and observed with a microscope at various magnifications.
Results: After a therapy based on a 500 mg clarithromycin tablet administered every 12 hours for 30 days as systemic therapy, a complete recovery of the patient from left eye inflammation was observed and SEM cytology showed that NTM colonies had disappeared.
Conclusion: Conjunctival cytology scraping and SEM technologies can be therefore exploited as new tools in diagnosis and fast identification of these newly discovered mycobacteria. In fact, they have a new way for studying ocular pathology, because of the simple execution and remarkable accuracy in the diagnosis. In fact, this technique allows to gather valuable information about all pathogens expression and the cellular action involved in pathology. As a further plus, this technique provides clinicians with the opportunity to repeat the SEM cytology for monitoring patients during therapy, hence leading to evaluate the efficacy of the pharmaceutical regimen in real time.
Nontuberculous mycobacteria (NTM) are microorganisms having higher chances to survive as compared to other bacteria and aerosol is the most common form of infection. NTM are ubiquitous and proliferate in conditions of extreme variability of temperature, pH and salt concentration and are detected in as many as environments, from contact lens washing solutions to aerosol devices, with an increasing culture time of up to 30 days. NTM infect immune compromised subjects as opportunistic and non-parasitic pathogens as opposed to tuberculosis mycobacteria that are obliged pathogens, instead [1,2]. According to the American Thoracic Society (ATS), NTM, known as pulmonary infectious agents, are a new class of saprophytes affecting biomembranes such as human eye. They specifically target immunodepressed patients using contact lenses that can alter tear film by reducing mucus, and therefore they can cause dacryocystitis, canaliculitis, conjunctivitis, scleritis, endophthalmitis, and keratitis of human eye and are more difficult than others to identify [3,4]. Their size ranges from 0.2 µm to 0.6 µm and, under a Ziehl–Neelsen (ZN) stain, they appear as red bodies on a blue background. They can be isolated in specific culture media, such as Lowenstein Jensen Medium, Middlebrook 7H9, from seven to fourteen days of culturing. NTM can be also currently identified thought other techniques such as Polymerase Chain Reaction (PCR) or on p-nitro-a-acetylamino-b-hydroxypropiophenone (NAP) method, usually performed in four to six days [4-6]. Anyhow, another technique that has been widely and successfully used to identify NTM, is Confocal Scanning Laser Microscopy (CSLM) system, that show NTM colonies with an “egg-fried”-like appearance. On the other hand, Scansion electronic microscopy (SEM) [7], allows to detect NTM colonies as single bacterium along with other bacteria, as spheroidal elements with a diameter ranging from 0.1 to 0.6 μm, often grouped in circular or domed aggregates or even as a cluster [8,9]. This short communication is aimed at demonstrating that scraping cytology and SEM can successfully assist in the diagnosis of NTM infection.
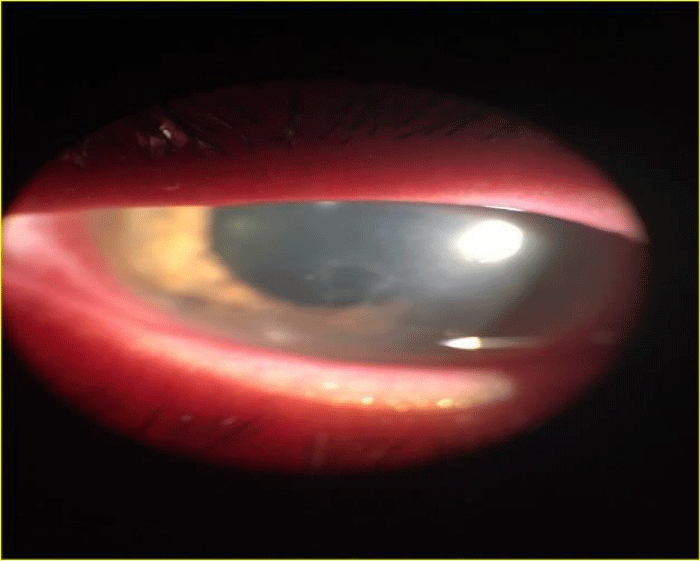
An Italian man of 19 years, habitual wearer of contact lenses, was admitted to our Department complaining pain and slit lamp examination showed infective corneal ulcer the of left eye (Figure 1). Objective signs: purulent secretion, conjunctival hyperemia, conjunctival pain; the patient referred he has been treated with other antibiotics such as quinolones and aminoglycosides but with no results. At our observation, the patient had visual acuity of 1/10 in left eye and 6/10 in right eye, assessed with a decimal system eye test card (Sbisà, Firenze). The patient showed an intraocular pressure (IOP) about 19 mmHg, in both eyes. The IOP was assessed by a Nidek NT 2000 auto noncontact Tonometer. Conjunctival cytological sampling and SEM were performed to confirm the diagnosis of infection disease caused by NTM.
Figure 1: Corneal inflammation of left eye (TOPCON SL7-F indirect ophthalmoscope).
The sampling was conducted according to the World Medical Association Declaration of Helsinki. Cytological samples were achieved by scraping technique, i.e. by crawling 2-3 times with a spatula on the mucosa, both sub palpebral and temporal area of the eye tarsal conjunctiva. The obtained sample was affixed to a sanded rectangular slide (Chemil s.r.l.) and stained with the Pappenheim method (3 min in pure May-Grunwald dye [Carlo Erba, Milan, Italy], washed in bidistilled water, treated for 8 min in Giemsa solution [Carlo Erba, Milan, Italy] (diluted 1:10 v/v), washed again and subsequently dried on a hot plate OTS 40. The sample was covered with Menzel –glasser cover slips 24 x 40 mm, one cover glass and observed with a microscope (Nikon Eclipse 50i) at various magnifications. The images were obtained with a Nikon ED 50F camera and digitized by computer software. For SEM, the samples placed on a 13 DIA (Agar scientific) circular slider (13 mm i.d.) were fixed in 2% glutaraldehyde, washed three times with 0.065M phosphate buffer pH 7.4, then placed in 4% OsO4. Samples were dehydrated through a graded (from 30% to 100%) solution of ethanol and dried by CO2 with Bemar SPC 1500 equipment. Specimens were mounted on a stub, sputtered in gold with SC7620, and observed with SEM JEOL JSM 5300; data were acquired by SemAfore software.
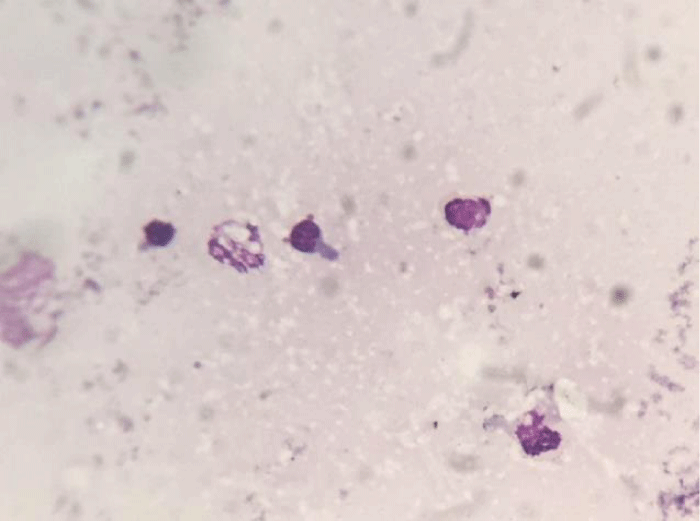
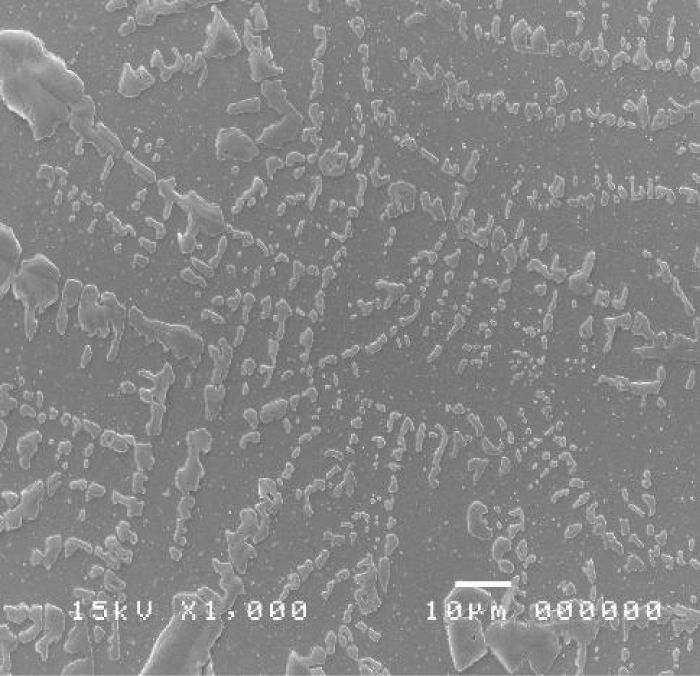
The cytological sample showed an infection, due to the presence of lymphocytes and polymorphonucleate cells (Figure 2). Numerous flaking cells were observed, but no bacterial colonies were observed since this test is unspecific (May Grunwald-Giemsa); instead, SEM technique allowed to observe the bacterial colonies colonizing the sample, detecting a pathogenic action towards the corneal conjunctival mucosa (Figure 3).
Figure 2: Cytological sample of left eye.
Figure 3: SEM image of left eye showing colonies of bacteria and NTM colonies.
Therefore, the patient was treated for both eyes for prevention with tetracycline eye drops (one drop for each eye every 2 hours) and tetracycline ointment (one time in the evening) for 30 days.
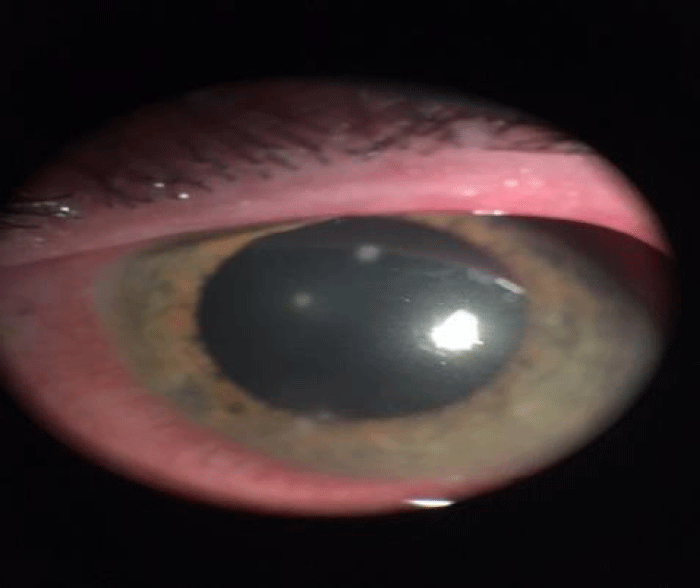
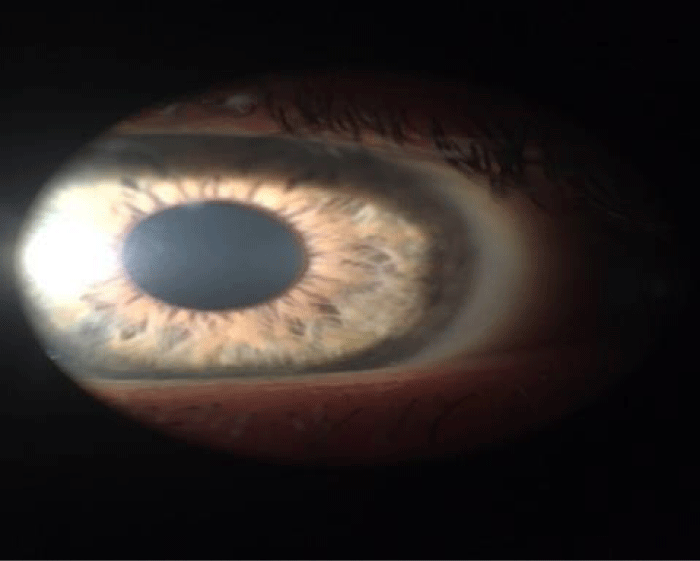
One 500 mg clarithromycin tablet was administered every 12 hours for 15 days as systemic therapy and lactic bacteria were also supplied to restore the intestinal bacterial flora. After fifteen days of therapy, the patient left eye’s ulcers healed and corneal infiltrates vanished (Figure 4). Since SEM technique confirmed colonies mycobacteria in left eye (Figure 5A,B), a further cytological sampling was carried out and the therapy was prolonged for other fifteen days, according to the protocol. After this further therapy course, a complete recovery from left eye inflammation was observed of the patient and SEM cytology showed that NTM colonies disappeared (Figures 6,7).
Figure 4: Corneal inflammation of left eye (TOPCON SL7-F indirect ophthalmoscope).
Figure 5: A,B. SEM images of left eye after the first course of therapy showing NTM colonies.
Figure 6: The healing of left eye ulcer and absence of inflammation with any spots.
Figure 7: SEM image of left eye showing no NTM colonies in the sample and Ferning test.
This research supports the use of the conjunctival scraping cytology and SEM technique in comparison with the application of a confocal that is the laboratory one or the in vivo Nidek/HRT type. Indeed, the latter cannot give the size of the pathogens to identify them with precision and/or effectiveness. The new diagnostic proposed procedure is relevant because it allows the identification of NTM pathogens that otherwise would not have been found with classic method (culturing of thirty days in specific medium and optical microscopy or confocal microscopy).
In fact, as previously stated, the drawback of NTM identification technique is that requires a long time to be performed and specific culture media that do not allow a fast diagnosis [10,11]. The time needed for diagnosis is crucial to avoid a serious infection degeneration. Scraping cytology and SEM can be performed in only two days, making this technique a good choice for quick diagnosis, and, moreover, being able to detect even few bacteria. SEM cytology is a fast technique with higher patient compliance and as such it could be considered as an election method for differential diagnosis. The use of SEM cytology becomes discriminating in therapeutic screening. Although an apparent healing of the corneal ulcer was assessed in figure 4 and 5A,B via ophthalmoscope observation. Figure 5A,B of the SEM cytology showed a persistence of the pathogenic agents, whose presence would have not been detected and the therapy addressed accordingly, causing recurrences. Conjunctival cytology scraping and SEM technologies pave a new way for studying ocular pathology, because of the simple execution and remarkable accuracy in the diagnosis. In fact, this technique allows to gather valuable information about all pathogens expression and the cellular action involved in pathology. As a further plus, this technique gives the opportunity to repeat the SEM cytology for monitoring patients during therapy, hence leading to evaluate the efficacy of the pharmaceutical regimen in real time.
The authors are grateful to Service Biotech laboratories and the Department of Neurosciences and Reproductive and Dentistry Sciences, University of Naples Federico II.
- Henkle E, Winthrop K. Nontuberculous Mycobacteria Infections in Immunosuppressed. Hosts. Clin Chest Med. 2015; 36: 91–99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25676522
- Thoracic.org.https://www.thoracic.org/statements/resources/mtpi/nontuberculous-mycobacterial-diseases.pdf.
- Henry CR, Flynn HW, Mille D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012; 119: 2443-2449. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22858123
- Hernandez-Toloza JE, Rincon-Serrano Mde P, Celis-Bustos YA, Aguillon CI. Identification of mycobacteria to the species level by molecular methods in the Public Health Laboratory of Bogota, Colombia. Enferm Infecc Microbiol Clin. 2016; 34: 17-22.
- Yam WC, Siu KH. Rapid identification of mycobacteria and rapid detection of drug resistance in Mycobacterium tuberculosis in cultured isolates and in respiratory specimens. Methods Mol Biol. 2013; 943: 171-99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23104290
- Morgan MA, Doerr KA, Hempel HO, Goodman NL, Roberts GD. Evaluation of the p-nitro-alpha-acetylamino-beta-hydroxypropiophenone differential test for identification of Mycobacterium tuberculosis complex. J Clin Microbiol 1985; 21: 634-635. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC271735/
- Cennamo GL, Del Prete A, Forte R, Cafiero G, Del Prete S, et al. Impression cytology with scanning electron microscopy: a new method in the study of conjunctival microvilli. Eye. 2008; 22: 138-143. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17603470
- Golding CG, Lamboo LL, Beniac DR, Booth TF. The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci Rep. 2016; 6: 26516. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27212232
- Carter HW. Clinical applications of scanning electron microscopy (SEM) in North America with emphasis on SEM's role in comparative microscopy. Scan Electron Microsc. 1980; 115-120. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7414259
- Set R, Shastri J. Laboratory aspects of clinically significant rapidly growing mycobacteria. Indian J Med Microbiol. 2011; 29: 343-352. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22120792
- Bittner MJ, Preheim LC. Other Slow-Growing Nontuberculous Mycobacteria. Microbiol Spectr. 2016; 4. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27837745