More Information
Submitted: 03 March 2020 | Approved: 07 April 2020 | Published: 09 April 2020
How to cite this article: Solomou A, Kraniotis P, Patriarcheas V. The role of Diffusion-Weighted Imaging in better delineating the extent of Diffuse Axonal Injury in a pediatric patient: A case report and brief review of the literature. Arch Case Rep. 2020; 4: 022-025.
DOI: 10.29328/journal.acr.1001034
ORCiD: orcid.org/0000-0002-8501-1192
Copyright License: © 2020 Solomou A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diffuse axonal injury; Diffusion-weighted imaging; Pediatric head trauma
Abbreviations: CT: Computed Tomography; MRI: Magnetic Resonance Imaging; DAI: Diffuse Axonal Injury; DWI: Diffusion-Weighted Imaging; ADC: Apparent Diffusion Coefficient; T2WI: T2-Weighted Image; T1WI: T1-Weighted Image; FLAIR: Fluid Attenuation Inversion Recovery; ICU: Intensive Care Unit
The role of Diffusion-Weighted Imaging in better delineating the extent of Diffuse Axonal Injury in a pediatric patient: A case report and brief review of the literature
Aikaterini Solomou*, Pantelis Kraniotis and Vasileios Patriarcheas
Department of Radiology, University Hospital of Patras, Rion, PC 26504, Greece
*Address for Correspondence: Solomou A, Department of Radiology, MRI Unit, University Hospital of Patras, Greece, Tel: 00306945904086; Email: solomou@med.upatras.grr
Introduction: Diffuse axonal injury (DAI) is a major cause of disability in the pediatric patient. Herein we describe the MRI/DWI findings in a case with DAI. We also discuss the current role of CT and MRI with DWI in the evaluation of DAI.
Aim of the study: To stress the role of diffusion-weighted imaging in diffuse axonal injury.
Methods: A pediatric patient, who was hospitalized in the ICU, was submitted to MRI with DWI for the evaluation of brain lesions. The patient was scanned with T1-weighted images, T2-weighted images, FLAIR, T2*-weighted images and diffusion weighted images.
Result: Brain lesions caused by DAI were more conspicuous on diffusion-weighted images compared to FLAIR images. T2*-weighted images were a helpful adjunct in showing micro-hemorrhages.
Conclusion: T2*-weighted images and FLAIR images alone underestimate the true extent brain lesions in DAI compared to DWI.
Traumatic Brain Injury (TBI), is a common healthcare problem, a significant cause of injury and mortality, affecting almost 10 million people annually. In pediatrics, TBI is the leading cause of death and disability with a variable clinical manifestation depending on the severity of trauma.
TBI can be classified as mild, moderate or severe according to the GCS score recorded during patients’ admission to the ER department, the duration of loss or alteration of consciousness and post-traumatic amnesia [1].
Proceeding to a neuroimaging study in patients with TBI is essential, because it plays a critical role in both diagnosis and evaluation of these patients. Imaging findings are strongly correlated with future outcome in terms of neurological deficits as well as long term developmental and mental defects [2,3].
Imaging findings are classified into focal and diffuse injuries.
Focal injuries arising from collision forces comprise epidural or subdural hematomas, intraparenchymal hematomas and cerebral contusions.
Diffuse brain injury, resulting from rapid acceleration-deceleration of the head, is more severe than focal injuries and includes DAI and diffuse brain edema. Diffuse brain injury is usually associated with the poorest outcome [4-6].
In a pediatric brain trauma series, the incidence of DAI was only 3.5% being the least common radiological feature among patients. The most common radiological features were skull fracture (51.2%), epidural hemorrhage (31.3%) and subdural hemorrhage (17.6%) [7].
As mentioned previously DAI is the least common consequence of brain trauma, it bears however a heavier toll to the afflicted patient. Imaging the extent of DAI lesions is crucial to patient’s outcome. Conventional MRI sequences (T2-weighted images and FLAIR) underestimate brain lesions in DAI, compared to diffusion-weighted imaging. We herein stress the role of DWI as the single best sequence in imaging these cases when it comes to MRI.
Seven year old boy sustained brain injury after the collapse of a bulky metallic object on its head and necessitated intubation. CT scan revealed bilateral fractures of the temporal bones without intracranial hemorrhage or midline shift.
The child was hospitalized in the pediatric ICU and was treated according to the head injury protocol, including the insertion of intracranial pressure (ICP) monitor catheter. During hospitalization, ICP measurements remained within normal limits, permitting sedation interruption and extubation. Due to abnormal neurological examination (diminished unilateral mobility and absence of response to commands), the patient underwent brain MRI. The patient was extubated the 11th day of hospitalization and admitted to the rehabilitation centre, showing a progressive improvement.
The patient was submitted to MRI using:
- Coronal, axial and sagittal FLAIR(TR8000msec/TE120msec) and slice thickness 4.5mm
- Axial DWI and ADC map(TR480msec/TE11msec) with b0 and b1000 mm2/sec and slice thickness 5mm
- Axial T1(TR228msec/TE4.8msec) and slice thickness 5mm
- Axial T2(TR4430msec/TE78msec) and slice thickness 5mm
MRI demonstrated abnormal signal intensity that was more extensive in the DWI images than in T2WI/FLAIR.
Lesions were hyperintense on FLAIR and T2-WI. Micro hemorrhage was detected as low signal punctuate foci on T2*-WI. On DWI, the lesions were hyperintense and on the corresponding ADC map images the lesions were hypointense, in keeping with true diffusion restriction. Abnormal signal mainly involved the brain hemispheres (subcortical and deep white matter) and to a lesser extent the corpus callosum (Figures 1-4).
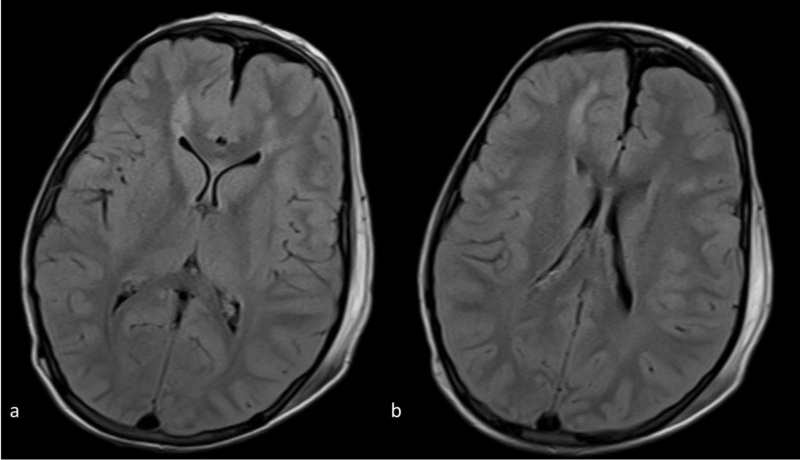
Figure 1 a,b: Axial FLAIR images at the level of the frontal horns (a) and bodies(b) of the lateral ventricles. There is abnormal signal intensity in the subcortical and deep white matter and a suspicious punctuate lesion in the genu of the corpus callosum.
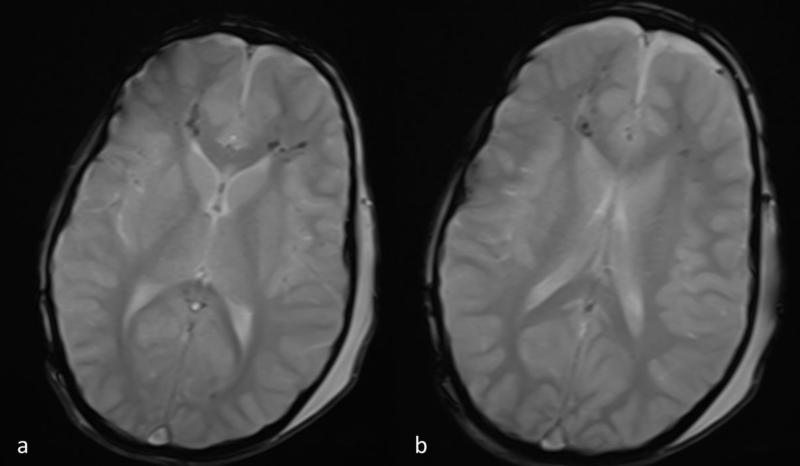
Figure 2 a,b: Axial T2*-weighted images at the same level as 1a,b. Hypointense blooming artifact is depicted in the regions of abnormal FLAIR signal, consistent with micro-hemorrhages.
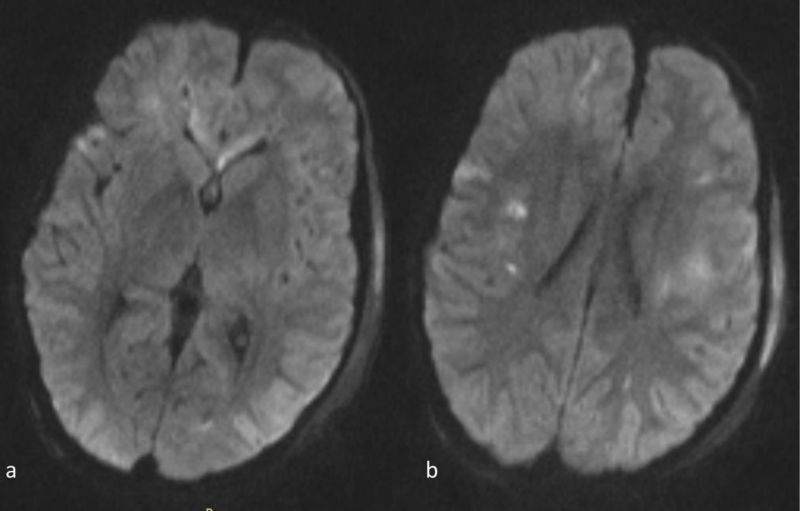
Figure 3 a,b: Axial DWI images at the same level as 1a,b. The abnormal signal intensity in the genu of the corpus callosum and the subcortical and deep white matter is more conspicuous than on the FLAIR images. Note also that the abnormal signal intensity with hyperintense lesions extends bilaterally far beyond the areas of abnormal signal on the FLAIR images(compare to 1a-b).
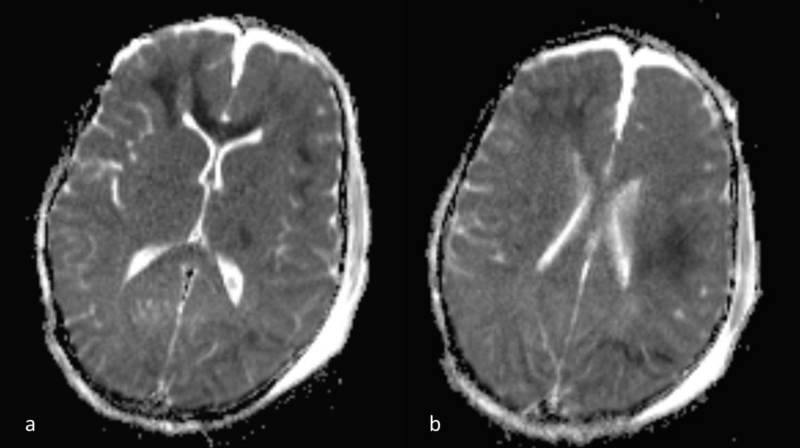
Figure 4 a,b: a) ADC map images, corresponding to 3a,b. The lesion hypointensity confirms that there is true diffusion restriction. DWI far better depicts the extent of DAI, compared to FLAIR alone (see figure 1). Note also the presence of small parafalcine and bilateral frontal hygromas.
Non-contrast Computed Tomography scan is the gold standard method for the initial investigation of brain trauma, as it is a fast and widely available modality [8,9]. The most common abnormal CT findings include hemorrhagic (hyperdense) lesions such as extra-axial hemorrhage (EDH, SDH, SAH), intra-axial (i.e. intraparenchymal) hemorrhage and intraventricular(IVH) hemorrhage or non-hemorrhagic (hypodense) lesions.
Diffuse brain injuries can be evaluated and classified by the Marshall classification of traumatic brain injury (MCTC) system, proposed by Marshall, et al. [10]. The MCTC system evaluates three imaging findings: Mesencephalic cistern, midline shift, high/mixed density mass lesions. Patients are categorized into six different groups with higher categories having worse prognosis and survival rate.
Many years later, MCTC remains one of the commonest used prediction systems [5]. However, it exhibits limitations such as:
- failing to distinguish between subtypes of intracranial lesions
- does not evaluate for DAI
A more recent system of TBI classification, the Rotterdam CT Score, developed by Mass, et al. [11] has been suggested, to overcome limitations of the Marshall system.
Rotterdam CT score evaluates four parameters:
The degree of basal cistern compression, the midline shift, the presence of epidural hematomas and the presence of SAH or intraventricular hemorrhage. Higher scores are associated with an increased probability of an unfavorable outcome [12].
However, a negative CT scan cannot rule out DAI and Magnetic resonance imaging (MRI) is more sensitive for the diagnosis of suspected DAI [9].
Apart from its higher sensitivity for the detection of DAI, MRI has also the ability to detect brain edema and micro-hemorrhages [13], thus providing important information for long term survival and outcome [14].
Taking into account that the majority of patients with DAI are younger patients and children, accurate diagnosis and prognosis is very important and should be considered a sine qua non for an improved therapeutic plan. Indeed a survey, by Ferrazzano, et al. [15] showed that MRI is nowadays more commonly obtained during the acute clinical setting in severe pediatric DAI.
In cases of DAI, MRI may more specifically demonstrate small regions of susceptibility artifact at the grey-white matter junction, in the corpus callosum or the brain stem. In the acute phase, MRI may show high signal on FLAIR sequences of the injured axons due to edema, whereas in the chronic phase due to neuronal loss, lesions compatible with Wallerian degeneration may be visible.
T1 and T2-WI are essential for the temporal detection of hemorrhage [16,17], but underestimate the extent of DAI [18,19].
T2*-WI detect hemorrhage in 10%–30% of DAI patients, correlating well with the unconsciousness period [20].
FLAIR may detect many non-hemorrhagic lesions in DAI [21-23], but FLAIR and T2*-WI underrate the extent of axonal injury, within the first 48 h [24].
The ADC value is frequently decreased in acute axonal injury, due to reduced water diffusion, but may increase or stay unchanged in some cases [24].
Reduced ADC values may be caused either by trauma-induced ischemia and/or cytoskeleton collapse due to axotomy [25,26]. ADC values are affected by susceptibility effects [27].
Within the first few days [28], restricted diffusion is found in brain regions surrounding focal lesions, while interestingly these regions show no abnormal signal in T2-WI. The extent of DWI lesions, not visible with FLAIR and T2*-WI, correlates with better clinical results [29,30].
DWI is of added value for better mapping the extent of lesions in DAI and correlates well with the clinical outcome, affecting patient management in the acute phase [29].
Our results are consistent with previous reports in the literature. DWI answered the question why a patient may not be performing well while there are confined lesions on FLAIR imaging. This is because FLAIR alone underestimates the extent of brain lesions. DWI may not be readily available in all hospitals around the world; if available however it should be applied, as valuable information may be either wise missed.
Although being the least common brain trauma consequence, DAI has a significant impact on the affected patients, in terms of neurological status and the need for rehabilitation.
The primary role of CT, in the pediatric trauma setting, is defined mainly to excluding acute hematomas, in need of surgical intervention.
On the other hand, MRI is crucial in depicting DAI, in cases where the initial CT is read as negative, but the patient is still in a severe neurological status. MRI correlates better with the final outcome, than CT alone. Referring to MRI, DWI is an indispensible supplemental tool for assessing the true extent of brain lesions in the pediatric patient with DAI.
- Levin HS, Diaz-Arrastia RR, Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015; 14: 506-517. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25801547
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007; 22: 341-353. Pubmed: https://www.ncbi.nlm.nih.gov/pubmed/18162698
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017; 66: 1-16. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28301451
- Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, et al. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma. 2011; 28: 701-709. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21401319
- Lolli V, Pezzullo M, Delpierre I, Sadeghi N. MDCT imaging of traumatic brain injury. Br J Radiol. 2016; 89: 20150849. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26607650
- McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015; 127: 45-66. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25702209
- Jeong HW, Choi SW, Youm JY, Lim JW, Kwon HJ., et al. Mortality and Epidemiology in 256 Cases of Pediatric Traumatic Brain Injury: Korean Neuro-Trauma Data Bank System (KNTDBS) 2010-2014. J Korean Neurosurg Soc. 2017; 60: 710-716. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29142631
- Le TH, Gean AD. Gean, Neuroimaging of traumatic brain injury. Mt Sinai J Med. 2009; 76: 145-162. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19306377
- Lee B1, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005; 2: 372-383. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15897957
- Marshall LF, Marshall SB, Klauber MR, Van Berkum Clark M, Eisenberg H, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992; 9 Suppl 1: S287-292. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1588618
- Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005; 57: 1173-1182. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16331165
- Charry JD, Falla JD, Ochoa JD, Pinzón MA, Tejada JH, et al. External Validation of the Rotterdam Computed Tomography Score in the Prediction of Mortality in Severe Traumatic Brain Injury. J Neurosci Rural Pract. 2017; 8 (Suppl 1): S23-S26. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28936067
- Amyot F, Arciniegas DB, Brazaitis MP, Curley KC6, Diaz-Arrastia R, et al. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J Neurotrauma. 2015; 32: 1693-721. PubMed: https://pubmed.ncbi.nlm.nih.gov/26176603/
- Haghbayan H, Boutin A, Laflamme M, Lauzier F, Shemilt M, et al., The Prognostic Value of MRI in Moderate and Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit Care Med. 2017; 45: e1280-e1288. PubMed: https://pubmed.ncbi.nlm.nih.gov/29028764/
- Ferrazzano PA, Rosario BL, Wisniewski SR, Shafi NI, Siefkes HM, et al., Use of magnetic resonance imaging in severe pediatric traumatic brain injury: assessment of current practice. J Neurosurg Pediatr. 2019; 23: 471-479. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6687576/
- Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, et al. Imaging of closed head injury. Radiology. 1994; 191: 1-17. PubMed: https://pubmed.ncbi.nlm.nih.gov/8134551/
- Parizel PM, Van Goethem OJW, van den Hauwe L, Dillen C, Verlooy J, et al. Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol. 1998; 8: 960-965. PubMed: https://pubmed.ncbi.nlm.nih.gov/9683701/
- Anderson CV, Wood DM, Bigler ED, Blatter DD. Lesion volume, injury severity, and thalamic integrity following head injury. J Neurotrauma. 1996; 13: 59-65. PubMed: https://pubmed.ncbi.nlm.nih.gov/8714861/
- Gale SD, Johnson SC, Bigler ED, Blatter DD. Trauma-induced degenerative changes in brain injury: a morphometric analysis of three patients with preinjury and postinjury MR scans. J Neurotrauma. 1995; 12: 151-158. PubMed: https://pubmed.ncbi.nlm.nih.gov/7629861/
- Yanagawa Y, Tsushima Y, Tokumaru A, Un-no Y, Sakamoto T, et al. A quantitative analysis of head injury using T2*-weighted gradient-echo imaging. J Trauma. 2000; 49: 272-7. PubMed: https://pubmed.ncbi.nlm.nih.gov/10963538/
- Ashikaga R, Araki Y, Ishida O. MRI of head injury using FLAIR. Neuroradiology. 1997; 39: 239-242. PubMed: https://pubmed.ncbi.nlm.nih.gov/9144669/
- Hesselink JR, Dowd CF, Healy ME, Hajek P, Baker LL, et al. MR imaging of brain contusions: a comparative study with CT. AJR Am J Roentgenol. 1988L 150: 1133-1142. PubMed: https://pubmed.ncbi.nlm.nih.gov/3258718/
- Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994; 15: 1583-1589. PubMed: https://pubmed.ncbi.nlm.nih.gov/7985582/
- Huisman TAGM, Sorensen AG, Hergan K, Gonzalez RG, Schaefer PW. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr. 2003; 27:: 5-11. PubMed: https://pubmed.ncbi.nlm.nih.gov/12544235/
- Ito J, Marmarou A, Barzó P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg. 1996; 84: 97-103. PubMed: https://pubmed.ncbi.nlm.nih.gov/8613843/
- Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995; 12: 555-564. PubMed: https://pubmed.ncbi.nlm.nih.gov/8683606/
- Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000; 217: 331-345. PubMed: https://pubmed.ncbi.nlm.nih.gov/11058626/
- Jones DK, Dardis R, Ervine M, Horsfield MA, Jeffree M, et al. Cluster analysis of diffusion tensor magnetic resonance images in human head injury. Neurosurgery. 2000; 47: 306-313; discussion 313-4. PubMed: https://pubmed.ncbi.nlm.nih.gov/10942003/
- Ezaki Y, Tsutsumi K, Morikawa M, Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol. 2006; 47: 733-740. PubMed: https://pubmed.ncbi.nlm.nih.gov/16950714/
- Schaefer PW, Huisman TA, Sorensen AG, Gonzalez RG, Schwamm LH. Diffusion-weighted MR imaging in closed head injury: high correlation with initial glasgow coma scale score and score on modified Rankin scale at discharge. Radiology. 2004; 233: 58-66. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15304663